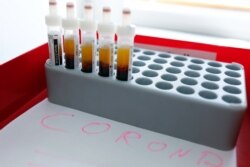
Only 2,000 frontline medical staff in Britain have been tested for coronavirus — to the frustration of hard-pressed doctors and nurses — and the country is struggling to test about 8,000 patients daily for the potentially deadly virus.
Meanwhile, Germany is managing to test about 25,000 patients a day and is ramping up testing with the country’s laboratories, which now are capable of conducting up to 500,000 COVID-19 tests a week.
Widespread testing is being credited for helping Germany to keep its coronavirus case fatality rate at a low 0.9 percent.
Why the discrepancy between two of Europe’s most powerful political and economic powers?
The answer, according to some officials and public health analysts, comes down to a more streamlined and collaborative partnership between business and government in Germany.
They highlight how quickly German virologists—working with the country’s public and private sectors —were able to respond rapidly in January to the looming threat of coronavirus by developing one of the first reliable methods for COVID-19 testing.
The German virologists were left largely unhampered by a restrictive central regulatory body second-guessing them or imposing burdensome procedures.
The German test was quickly adopted by the World Health Organization, which offered to supply test-kits to other countries.
Britain, like the United States, didn’t take up the offer, preferring instead to develop their own testing methods for the virus. In the meantime, while they did that, private German manufacturers moved quickly to mass produce test-kits, allowing Germany to get a head-start.
Britain and the U.S. have been “playing catch-up ever since,” says Jeffrey Singer, an Arizona physician and public health analyst at the Cato Institute, a libertarian think tank based in Washington.
London and Washington have “failed to make use of the innovation, flexibility and speed of the private sector,” he says. The Food and Drug Administration required an onerous approval process to bring any test to market and withheld approval of tests developed both by commercial enterprises and universities.
Instead, it authorized the Centers for Disease Control and Prevention to develop a coronavirus test, and the kits on the first rollout were faulty, Singer says.
“The CDC took control of distributing and administering tests, while the private sector and foreign-developed tests were kept out of the process during the crucial weeks between when the virus was first identified in December and when it started rapidly spreading among the American public,” Singer adds.
Only belatedly have the obstacles to private-sector testing been lifted in the U.S.
For Singer and some other public health analysts, the testing missteps in the U.S. and Britain are emblematic of a failure to fully enlist the private sector in the battle against the coronavirus.
That’s a surprising development since both countries are viewed as bastions of free enterprise, more so even than Germany — or South Korea and Australia, which also have seen much quicker rollouts of mass testing thanks to successful public-private partnerships.
“We should be relying more on private enterprise and it is shameful we aren’t,” Singer says.
His complaint is echoed in other European countries, including Britain, where hospitals have been pleading for testing supplies in order to ferment their own reagents, the specialist chemicals needed for coronavirus testing. Some public health service labs are only able to carry out two or three a day because they are short of swabs, while others are at a standstill because of the shortage of reagents, according to hospital chiefs.
As the coronavirus death toll rose midweek to 2,352, ministers have faced mounting public criticism for the delays and missteps in ramping up testing — as well as delays in approving innovative, cost-effective ventilators that could make up a critical shortfall. A spin-off diagnostic unit of the University of Cambridge only belatedly has secured validation for a portable testing method that can provide results in 90 minutes.
Ministers in the ruling Conservative government blame a global shortage of reagents for the delay in boosting testing, and British Prime Minister Boris Johnson has placed himself in charge of finding supplies. The failure to ramp up testing is turning into a political crisis. Conservative-leaning newspapers now have turned on the government, with The Times saying virus testing plans were in “chaos.”
Critics say the government needs to relax the regulatory rules that govern which laboratories can conduct tests.
There currently is a highly controlled centralized approach shutting out commercial labs. But more than a dozen British companies have developed new coronavirus tests that use different and readily available reagents. They are not allowed to proceed until the accuracy of their testing methods are assessed and accredited by the public health service, which is taking time.
In support of the government’s position, Alex Blackmore, a life sciences professor at Britain’s Brunel University, told media outlets, “The problem with a lot of molecular biology is that if you don’t do it well, it can be difficult to interpret results. You’ve got to decide which tests are valid and which aren’t. You’ve got to train people to use those tests specifically, and you’ve got to validate the laboratories.”
But Anthony Costello, a former director at the World Health Organization, says any public inquiry into Britain’s coronavirus response once the pandemic is over will “find a litany of failures.”
Among them, the failure to enlist the services of commercial labs. He told the BBC, “We have 44 molecular virology labs in the UK. If they were doing 400 tests a day, we would be up to Germany levels of testing and that is perfectly feasible.”
He says there has been a failure to move quickly and effectively — a symptom, he fears, “of a more comprehensive system failure.”
Talk of “system failure” is mounting not on in Britain, but also in Italy and Spain where businesses say their offers to assist in manufacturing protective gear for hospital workers and developing new, inexpensive ventilators often go unanswered or aren’t followed up on after discussions get under way.
In the U.S., businesses say they also encounter regulatory obstacles, which some put down to bureaucratic inertia, others to lack of imaginative thinking.
Ohio company Battelle has been trying for weeks to get FDA approval for an innovative system that can decontaminate 80,000 masks a day. Only after the intervention of Ohio Governor Mike DeWine, who appealed directly to the White House, were the regulatory objections overcome. Battelle got approval Sunday.
The current crisis “demonstrates a broken regulatory system that kills by delaying new tech and demanding paperwork for increasing medical capacity,” says Tom Palmer, a vice president at the Atlas Network, a non-profit that advocates for free-market economic policies.
“In the U.S., certificates of need have restricted creation of new clinics, installation of ventilators, and on and on. Four different agencies regulate respirators. It’s death by bureaucracy,” he adds.