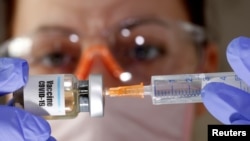
The competitive scramble for resources to fight the COVID-19 pandemic has advocates concerned that the countries with the biggest wallets will snatch up supplies and leave others behind.
It has happened already with personal protective equipment. In April, the Trump administration ordered maskmaker 3M to stop exporting N95 masks to Canada and Latin America.
On Monday, the U.S. Department of Health and Human Services announced it had secured nearly the entire supply of remdesivir, the first new anti-COVID-19 drug, from the manufacturer.
"This is a harbinger of bad things to come unless it's exposed and other ideas are put forward," said Northeastern University law professor Brook Baker, a senior policy analyst for Health Global Access Project.
First doses
Around the world, governments are scrambling to lock down access to COVID-19 vaccines that have not yet been proved safe or effective.
Pharmaceutical company Sanofi's Chief Executive Officer Paul Hudson told Bloomberg News that the $30 million the United States had invested in the company's vaccine meant Americans would get its doses first, if it works.
Hudson walked that statement back the next day after objections from the government of France, where Sanofi is headquartered.
In Britain, Business Secretary Alok Sharma said his country would get the first 30 million doses of a vaccine candidate under development by the University of Oxford and drugmaker AstraZeneca and would contribute $80 million to the effort.
Four days later, the U.S. government announced a $1.2 billion deal to secure 300 million doses of the same vaccine.
Companies have manufacturing capacity in multiple countries, so "we are in no way inhibiting the rest of the world from getting these same vaccines," a senior Trump administration official said during a June 16 conference call with reporters. Administration ground rules prohibit the official from being named.
The virus spread through international travel, the official acknowledged, but added, "Let's take care of Americans first. To the extent there is surplus, we have an interest in ensuring folks around the world are vaccinated."
Vaccine nationalism is not confined to the Western world.
The Serum Institute of India also has a deal to produce 60 million doses of the Oxford-AstraZeneca vaccine.
“A majority of the vaccine, at least initially, would have to go to our countrymen before it goes abroad,” managing director Cyrus Poonawalla told Reuters, though he said the Indian government would make the final decision.
Connected world
"This behavior is not new. We've seen this before in almost every pandemic," said Rebecca Weintraub, faculty director of the Global Health Delivery Project at Harvard University.
Rich countries preordered vaccines against H1N1 influenza during the 2009 pandemic, crowding out poor countries, she said. The wealthier countries eventually donated vaccines to lower-income countries, but only after they were sure they had enough for themselves.
But in a globally connected world, experts say, any disease is just a plane flight away.
"It really has to be understood that the least of the countries to be prepared presents a global health risk for all of us," said Thabani Maphosa, managing director of the country programs department at GAVI, the global vaccine initiative.
Global health authorities are working to put together a viable alternative to vaccine nationalism.
In late April, the World Health Organization launched the Access to COVID-19 Tools Accelerator to speed up development and equitable distribution of drugs, vaccines and tests.
"The advantage of this type of centralized allocation principle is we will end the pandemic faster," Harvard's Weintraub said. "We will return to a more vibrant economy."
Vaccine club
A lot of the details are still being worked out.
"This is a plane being built as it flies," Baker at Northeastern University said. But the structure is taking shape, he added.
The vaccine branch aims to act like a buyers' club. Countries put money into a common fund that backs development of several vaccines at once. Since most vaccines fail before they reach the market, supporting several is a way to hedge bets.
Having more than one vaccine means competition that would bring down the price, GAVI's Maphosa said. GAVI is co-leading the vaccine section of the ACT Accelerator.
It also gives manufacturers an idea of how much vaccine they need to make and how much infrastructure they need to commit, he added.
Countries pay into the fund based in part on the number of their most vulnerable citizens, including health care workers, the elderly and people with other health conditions that put them at higher risk.
Two-tiered system
There won't be enough vaccine initially for everyone who needs it. So, countries that pay into the fund will receive enough to immunize up to 20 percent of their populations.
Low-income countries will rely on donors to pick up the bill. These countries will receive vaccines based on a framework the WHO is developing to prioritize health care workers and the most vulnerable.
That two-tiered system concerns some advocates. The number of people covered in low-income countries will vary from country to country, but "it's certainly less than 20 percent of the population," Baker said.
The ACT Accelerator aims to supply 2 billion doses of vaccine by the end of 2021. Doses will be divided equally between countries that pay their own way and countries that donors cover.
But the population of low- and middle-income countries that will likely get donor support is much larger than that of higher-income countries that will pay their own way.
"If you do the math, that means that rich people will have five times the likelihood of access than people in poor countries," Baker said.
That's not as bad as it seems, GAVI's Maphosa said.
"Allocation of the vaccine is supposed to respond to the disease threat and to the vulnerabilities. At this particular point, the threat has been immense in the West," he said.
A Western country like Italy, which was ravaged by the virus, "has a very old population, and Africa has a very young population," he noted. "And this is a disease that is putting the old at risk."
The ACT Accelerator has secured pledges of $3.4 billion out of the $31.3 billion it estimates is needed for vaccines, treatments and tests.
The United States has not pledged financial support, though technical experts are contributing. President Donald Trump has said he aims to cut ties with the WHO over its response to the pandemic.
So far, one manufacturer, AstraZeneca, has announced plans to participate. The company says it will deliver 300 million doses by the end of the year.